Finding Acoels & Nemertodermatids:
Here are some videos on how to collect, extract and then treat specimens. First, sediment has to be collected - this can be done with different methods. For worm burrows in the intertidal, a "slurp gun" can be used. This video show how to make an extraction of meiofauna with magnesium chloride. After the extraction, we sort out acoels, and if we are lucky we find many of one species alone.
Classification:
The classification of Acoela down to the “family level” was revised based on a phylogenetic study (U. Jondelius, A. Wallberg, M. Hooge, and O. I. Raikova, “How the Worm Got its Pharynx: Phylogeny, Classification and Bayesian assessment of Character Evolution in Acoela,” Systematic Biology, 2011.doi:10.1093/sysbio/syr073 )
Here is an overview of the current acoel classification (with diagnostic features of higher taxa in brackets). The suprafamilial clade names are informal, i.e. not part of a linnean classification.
1. Acoela (digestive parenchyma, biflagellate spermatozoa)
1.1. Diopisthoporidae Westblad, 1940
1.2. Bitesticulata (Paired or follicular testes, ventral gonopore. The most inclusive clade that contains Paratomella rubra and Symsagittifera roscoffensis but not Diopisthoporus longitubus)
1.1.1. Paratomellidae Dörjes, 1966
1.1.2. Bursalia (Copulatory bursa often present. The most inclusive clade that contains Oligofilomorpha interstitiophilum and Childia groenlandica but not Paratomella rubra)
1.1.1.1. Prosopharyngida (Muscular pharynx in anterior part of body. The most inclusive clade that contains Hofstenia miamia and Oligofilomorpha interstitiophilum but not Haploposthia rubra)
1.1.1.1.1. Hallangidae Westblad, 1946
1.1.1.1.2. Hofsteniidae Papi, 1957
1.1.1.1.3. Solenofilomorphidae Dörjes, 1968
1.1.1.2. Crucimusculata (Ventral crossover muscle fibres). The most inclusive clade that contains Actinoposthia beklemischevi and Childia groenlandica but not Haploposthia rubra)
1.1.1.1.4. Dakuidae Hooge 2003
1.1.1.1.5. Isodiametridae Hooge & Tyler 2005
1.1.1.1.6. Otocelididae Westblad, 1948
1.1.1.1.7. Proporidae Graff, 1882
1.1.1.1.8. Aberrantospermata (Spermatozoa with 9+0 or 9+1 axonemes. The most inclusive clade that contains Neochildia fusca and Childia groenlandica but not Actinoposthia beklemischevi)
1.1.1.1.1.1. Convolutidae Graff, 1905
This family now includes species formerly classified in Anaperidae and Sagittiferidae, which have both been synonymized with Convolutidae
1.1.1.1.1.2. Mecynostomidae Dörjes, 1968 This family now includes the species formerly classified in Childiidae as that taxon was synonymized with Mecynostomidae
1.3. Acoela Incertae cedis, no phylogenetic hypothesis available
1.1.3. Actinoposthiidae (poorly sampled in the phylogenetic study, indications that this may be a polyphyletic assemblage)
1.1.4. Anthroposthiidae (single species)
1.1.5. Antigonariidae (single species)
1.1.6. Nadinidae (single species)
1.1.7. Tauridiidae (single species)
Anatomy:
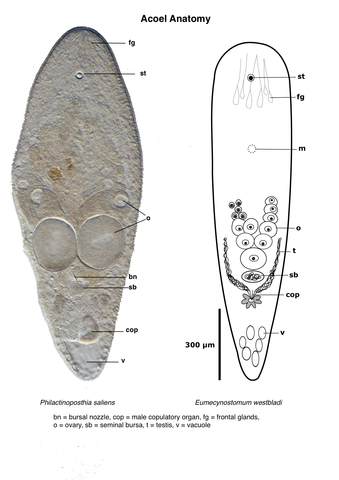
The statocyst with a single highly refractile statolith is located anteriorly and can often be seen in the dissecting microscope. In many species there are prominent glands that open through an anteroterminal pore; these are the frontal glands. In mature animals, eggs are easily detected as very large cells with a large nucleus. Discerning the testes usually requires higher magnification. They are normally located anterolaterally to the ovaries, but there are exceptions. There are many different types of male copulatory organs including a simple pore, a ciliated antrum, a muscular penis or a sclerotized stylet. A female gonopore is present in most, but not all species. Female accessory organs may be present in the form of a bursa for storage of allosperm. In some species the bursa is equipped with a bursal nozzle, a narrow passage through which allospermatozoa have to pass in order to fertilize the oocytes. Other features of importance for identification of acoels are pigment patterns, presence of a pharynx (in a small number of species), presence of symbiotic algae, and body shape and size.
Magnesium chloride (MgCl₂) extraction of acoels and other meiofauna:
Acoels are common in marine sediments such as sand and mud. The MgCl₂ method is well-suited for the extraction of acoels and other meiofauna from samples taken from a sandy beach or sublitorally. This video demonstrates how to perform a magnesium chloride extraction.
A brief history of acoel classification:
The name Acoela was introduced by Uljanin (1870) to denote free-living flatworms (“turbellarians”) without an intestinal lumen. An early, pre-cladistic classification of Acoela in the two families Proporidae (acoels with one gonopore) and Convolutidae (acoels with two gonopores) was proposed by von Graff (1905; 1911), who also elevated Acoela to the rank of subclass. The diagnoses of Convolutidae and Proporidae were modified by Luther (1912) to instead include acoels possessing a copulatory bursa (a secondary female organ for storage of allosperm) in Convolutidae and those lacking a copulatory bursa in Proporidae.
Einar Westblad studied the Swedish acoel fauna in a series of papers where he gave detailed accounts of their anatomy and proposed a comprehensive classification of Acoela, again working in a pre-cladistic framework (Westblad 1940, 1942, 1945, 1946, 1948). Westblad’s system was based on the characters that he perceived as the most important for the systematization of Acoela, namely the histology of the gonads, presence or absence of female copulatory organs and the position and anatomy of the male copulatory organ. Among these characters, Westblad identified several primitive states: follicular gonads, mixed male and female gonads, posterior male gonopore, and lack of copulatory organs. Other primitive states proposed by Westblad (1948) were an epithelial nervous system, and a ventral mouth without a pharynx. The families Diopisthoporidae, 1940, Haploposthiidae, Hallangidae, 1946, and Otocelididae, 1948 were introduced by Westblad in addition to Proporidae and Convolutidae. Papi (1957) introduced the family Hofsteniidae for Acoela with an exceptionally muscular pharynx. Paratomellidae was introduced by Dörjes (1966).
A major transformation of acoel taxonomy was carried out by Dörjes (1968), who named a large number of new species and introduced the families Anaperidae (antrum masculinum with more than one sclerotized prostatoid organ), Antigonariidae (monotypic family with a hermaphroditic mixed gonad), Childiidae (conical penis stylet), Mecynostomidae (spherical posterosubterminal male copulatory organ), Nadinidae (with large, partially ciliated pharynx, monotypic) and Solenofilomorphidae (slender acoels with pharynx and posterior ovary) as well as numerous new genera. None of these were based on explicit phylogenetic hypotheses.
Development after the publication of Dörjes 1968 monograph has been incremental. The monotypic family Taurididae was proposed by Kostenko (Kostenko 1989) based on a peculiar pattern of the male copulatory apparatus characterized by consecutive connection of the vesicula seminalis, vesicula granulorum and ciliated antrum. Kostenko and Mamkaev (1990) introduced the Sagittiferidae (saccate male antrum, epidermal extrusomes-sagittocysts). The configuration of the body wall musculature was emphasized as phylogenetically informative by Hooge and Tyler (Hooge 2001; Hooge 2003; Hooge and Tyler 2005) who introduced four new families: Actinoposthiidae, Hooge 2001, to which species with “normal” body wall musculature, i.e. circular muscles in the outer layer, were transferred from Childiidae; Dakuidae, Hooge 2003, a monotypic family with muscular seminal vesicle surrounding a bulbous penis; Polycanthiidae, Hooge 2003 again a monotypic family with multilayered muscular seminal vesicle and no penis; and finally Isodiametridae, Hooge and Tyler 2005 a large family of unpigmented acoels with an isodiametric penis invaginated into the seminal vesicle.
